Mood Disorders: How Understanding Underlying Mechanisms Enhances Patient Outcomes

There are a number of theories and models to explain the pathophysiological mechanisms underlying mood disorders. The biology is complex and varied, yet with stepping stones of concrete and comprehensible mechanisms. This could provide distinct targets for intervention. Our role as naturopathic doctors is by using these details like a broad template from which to understand the patient and find out the individualized road to homeostasis and wellness.
For several years, the vista of depression was dominated by a monoamine “chemical imbalance” hypothesis, mainly involving serotonergic and noradrenergic neurotransmission. However, just one and distinct mechanism for this simplified explanation of the complex illness is not discovered. Current published research paints an image that the monoamines are only 1 small bit of a bigger puzzle.1 Therefore, it is clear from research that mood disorders including depression ought to be “re-conceptualized” to include gross brain changes connected to stress and neuro-endo-immunological mechanisms.2
We know chronic illness is a mixture of contributing factors interacting to create a physiological adaptation. Depression is really a chronic disease initiated or perpetuated by a maladaptive neural, neuroendocrine, and immunological stress response. Four main areas of this method involve1:
- Cognitive processing
- Sympathetic activation and the hypothalamic-pituitary-adrenal axis
- Neuroplasticity and neurogenesis
- Inflammation and Immune activation
Cognitive Processing
The capability to consume new information in the environment, make sense of it, and respond accordingly is essential for healthy mood and cognition. Essential neurobiological mechanisms of cognitive processing have been shown to be dysfunctional in mood disorders. This involves the pre-frontal cortex , limbic system and, most importantly, the connections between these 2 areas. The PFC and limbic system have been in constant communication with each other, as well as their connections have been in constant flux. The structure is dynamic, even in the adult brain. It's the disruption of this communication – like 1 family member refusing to pay attention and the other shouting louder than could be heard – that disrupts overall cognitive processing adding to anxiety, depression, and other mood disorders.
The PFC is easily the most evolved part of the brain, with particular importance for executive function: planning, decision-making, controlling impulses, and motivation. The PFC could be further split into specialized areas of function which all play a vital role in emotional regulation and self-awareness. Deeper within the brain, the “feeling” limbic product is evolutionarily more ancient, and plays a huge role in raw emotions and unconscious experience: excitement, fear, anxiety, memory, and desire. This really is made up of 4 main regions: the hypothalamus, the amygdala, the hippocampus, and the cingulate cortex. Again, each serves a slightly different role in processing information and adding to a response: the hypothalamus controls stress, the amygdala accounts for extreme negative emotions such as fear and fright, the hippocampus controls the formation of long-term memories and calms the HPA, and the cingulate cortex plays a role in focus and a focus.3
Environmental stressors towards the body are transmitted towards the amygdala and cortex via the thalamus, activating the HPA system. Critical in mood disorders are the connections the amygdala has with parts of the PFC that play a role in emotional regulation and stress response inhibition, such as the orbitofrontal cortex , medial PFC, and anterior cingulate cortex . These areas then project to limbic structures like the amygdala and hypothalamus.1,4 Animal studies have indicated that these projections help control the amygdala and hypothalamus, modifying the strain response.5 In people with major depressive disorder , imaging research has revealed a low volume of the ACC, decreased activity of the dorsolateral PFC, and hyperactivity from the amygdala.6,7 This imbalance reflects a hyperactive HPA with diminished regulatory capacity – a physical obstacle to cognitively processing external and internal information and adequately inhibiting a stress response. Additionally, the hippocampus, responsible for cementing brand new memories, continues to be demonstrated to possess decreased volume and abnormal activity in depression.2 This data reflects the expertise of depression: daily activities producing painful stimuli in your body. This is because the neural structures and connections essential for emotional communication and also the formation of new memories show structural and functional impairment. This can be a physiological gap in mental and physical stress response management and observable pathology.
Sympathetic Activation & HPA axis
Activation from the HPA axis is initiated in limbic structures via indirect and direct projections to the hypothalamus, occurring without conscious awareness. A neuroendocrine cascade triggers the systemic discharge of glucocorticoids , DHEA, norepinephrine, and epinephrine. Multiple negative feedback loops exist to avoid this train from running from the tracks. They are contained in the hippocampus, hypothalamus, and anterior pituitary, and actually inhibit this HPA cascade. For instance, cortisol acts on the hippocampus, which in turn inhibits the hypothalamus with GABAergic projections.1
Short-term HPA activation increases arousal, cognitive functioning, and gluconeogenesis via catecholamines and glucocorticoids. However, long-term activation from the HPA system will ultimately dissolve the compensatory negative feedback loops, contributing to poor recovery and neuroendocrine imbalance. Globally, we may see disruptions in circadian rhythm that create fatigue, insomnia, and immune dysfunction. Molecularly, we have seen decreased expression of glucocorticoid receptors within the hypothalamus and hippocampus, so much so it has been declared “a key mechanism in the pathogenesis of depression” in the literature.8 This connection is so strong that Tafet and Nemeroff, in a 2023 overview of the hyperlinks between stress and depression, state that “an increasing body of evidence props up association between chronic stress and depression at the molecular level, where hyperactivity from the HPA axis, using the consequent increase of cortisol, represents one of the most consistent findings both in syndromal mood and certain panic disorders.”1,9 Additionally, evidence suggests a “U-curve” of glucocorticoid activity; that is, “dysregulation from the HPA axis towards both hyper- or hypoactivity manifesting like a risk to mental wellbeing.”10 This is reflective of the items we have seen clinically in the variability of both stress and mood patient-presentations and HPA biomarker testing. It's also reflective from the dynamic whole-system dysfunction that occurs inside a conventional diagnosis.
As stated above, patients with depression have consistently displayed statistically significant diminished hippocampal volume, demonstrating this important piece both in the negative feedback loop and cycle of depression as a complex chronic illness.11 In addition, hippocampal volumes have shown to have significant negative correlation with amount of depressive disorder, suggesting worsening hippocampal function with worsening illness.12 The HPA axis, sympathetic activation, and subsequent dysfunction of negative feedback loops demonstrate a vital bit of the complex puzzle of mood disorders, as well as key regions of intervention.
Neuroplasticity & Neurogenesis
Another section of research concentrate cognitive function, further expanding the image of mental health, is neurotrophic factors that contribute to not just brain development, but additionally neuroplasticity and neurogenesis in the adult brain. Brain-derived neurotrophic factor is probably the most well-known and well-researched neurotrophic factor. It stimulates cells within the hippocampus to proliferate and migrate to the left PFC, and is stored in platelets where it can facilitate neurogenesis in damaged tissues. Decreased expression of BDNF can bring about depression, while upregulation of BDNF has revealed a job in clinical recovery.13 Studies have discovered lower levels of BDNF within the serum of those diagnosed with MDD compared with healthy controls.14 Chronic amounts of glucocorticoids have been shown to induce neuronal cell damage and atrophy, impair neurogenesis in the hippocampus, and impair neuroplasticity.15 This means that there is clear cross-talk between cortisol and BDNF, and this relationship is central to both the pathogenesis and symptomatology of depression and mood disorders.
Inflammation & Immune Activation
Numerous studies in psychoneuroimmunology have taught us that an inflammatory fact is not just initiated by injuries and infection, but may also be activated both in acute and chronic psychosocial stress. The earliest proof of inflammation associated with major depression dates back to 1991.16 Since this time, “a vast literature has reproduced these bits of information, and meta-analyses have revealed that peripheral blood elevations in cytokines and acute-phase reactant are some of the most reliable biomarkers of increased inflammation in depressed patients,”16 in particular, interleukin -1β, tumor necrosis factor -α and C-reactive protein , respectively. It has been shown the increased inflammatory markers in depressed patients go back to control levels with successful antidepressant treatment.16 Furthermore, people who fail to respond to treatment show increased inflammatory markers.16 It's also extensively recorded that the medical administration of inflammatory cytokines for example interferon -α induces depressive symptoms.16 Those with depression also exhibit a greater immunological sensitivity to stress; eg, the strain of speaking in public has been shown to increase nuclear factor-κB .18 Clearly, immune dysfunction, including immune activation and inflammation, remains another bit of the complex puzzle of depression along with other mood disorders.
Psychosocial stress will activate the sympathetic nervous system and HPA axis. Long-term, this makes negative feedback loop disruption. We also know that psychosocial stress will activate the immune system in susceptible individuals. Another link within this cycle is the fact that, “psychosocial stressors are well-established precipitants of episodes of major depression.”16,17 Even though the impact from the sympathetic central nervous system around the defense mechanisms is complex and varied, it has been shown that catecholamines will “activate their receptors on immune cells” and stimulate the release of proinflammatory cytokines via the NF-κB cascade.1,18 This occurs mainly on macrophages and monocytes in peripheral blood.18
Prolonged increase of proinflammatory cytokines within the brain results in a reduction in neurotrophins for example BDNF, reduced neuronal repair, decreased neurogenesis, and activation of glutamatergic neurotransmission, resulting in apoptosis and oxidative stress.18 This diminishes ale the mind to take new information, make new connections and strengthen existing ones, and causes it to be hard for the person to perform the daily tasks that rely on these minds. This is especially true in relation to social and emotional processing. Figure 1 displays the relationship of those mechanisms within the cycle it makes. This cycle can be used a possible diagram of depression, showing how the patient's physiology becomes bound by these poorly-regulated compensatory mechanisms.
Teasing apart these pieces permits us to better understand the underlying causes of depression. This biological map of interdependent and functional nuances provides a general terrain by which to assess biomarkers and provide intervention to improve the dynamic ability from the human body to rebalance itself to homeostasis.
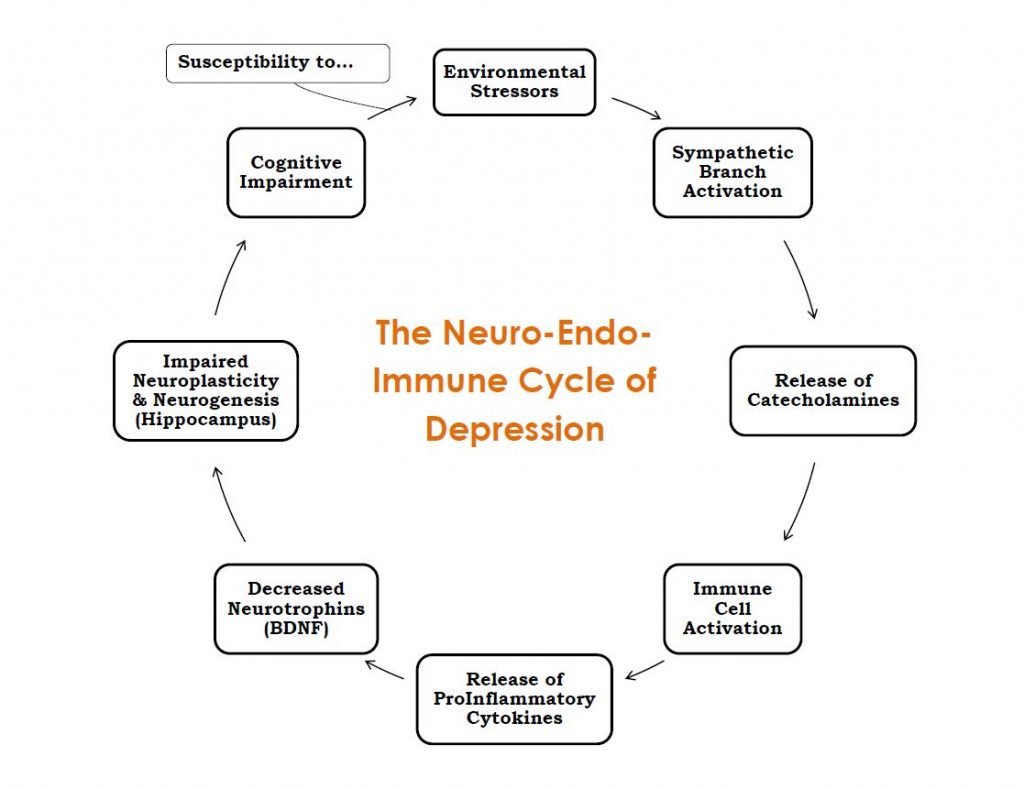
Figure 1. Neuro-Endo-Immune Cycle of Depression
Treatment Interventions
Since complex chronic diseases such as depression really are a selection of interrelated physiological mechanisms, addressing chronic disease is enhanced using the comprehensive and multi-modal approach of naturopathic medicine. While there is # 1 single remedy for depression, a mix of interventions specific to individualized dysfunction provides a path for improvement and also the best chance for recovery. This research is part of the road map that this path could be blazed.
Exercise
Due to the central importance in brain health, numerous investigators have checked out methods to increase BDNF, primarily by exercising. Animal studies have shown a rise in both BDNF gene expression and protein levels in the hippocampus having a daily exercise program.19 This is exactly the effect we would want to be able to provocke restore neurogenesis, neuroplasticity, and improve HPA regulation. Additional animal studies have shown that alternating days was competitive with daily exercise which BDNF remained elevated for several days after exercise ceased, suggesting the results to become a lot more than transiently palliative. Furthermore, animals with prior exercise experience show an immediate return to normal BDNF levels upon only brief reintroduction to exercise.19 This suggests that exercise primes the body to make BDNF at the molecular level.
This is proven in humans, and also the BDNF increase appears to differ across exercise program, gender, and – for ladies – timing with menstrual cycle.20 A 2023 review of 32 studies concluded that BDNF is increased with a single session of aerobic fitness exercise and further magnified with exercise frequency. The parameters of a “single session of aerobic exercise” ranged from 20 to 90 minutes at 75% of maximal heartbeat.20 The “frequency” range was 45 minutes 3 times per week for 3 months, to 60 minutes 5 times a week for six months, at 50-75% heart-rate reserve.20 This can be a reminder that setting specific parameters would prove more efficacious than the usual general recommendation. It also appears that these benefits are specific to aerobic fitness exercise. In this same review, the authors state that a “majority of the studies suggested that weight training didn't have affect on peripheral BDNF.”20
Minerals
In addition to exercise, the micronutrients zinc, magnesium, and lithium have all demonstrated a relationship with BDNF and depression.21 In animal studies, zinc has been shown to enhance BDNF gene expression, while zinc deficiency has been associated with low BDNF in humans.21
Curcumin
Curcumin is a low-molecular-weight polyphenol in Curcuma longa that is widely considered inexpensive, orally bioavailable, and highly safe in humans. Research on curcumin along with other curcuminoids is vast, with depression as a main focus point. The mechanisms because of its actions in depression are thought to be associated with monoaminergic activity, oxidative stress, HPA axis, and inflammatory pathways.22 More specifically, it has been shown to inhibit the monamine oxidase enzyme accountable for the introduction to serotonin, dopamine, and norephinephrine.23 Accordingly, it has also been proven to modulate the amount of these neurotransmitters necessary to affective and cognitive function.23 In animal studies, curcumin has been shown to increase levels of BDNF and promote hippocampal neurogenesis.23
Where it's most well known and well researched is as an anti-inflammatory agent. Curcumin will inhibit the isoenzyme cyclooxygenase-2 , NF-κB, and block the synthesis from the nitric oxide synthase enzyme.23 It has also been shown to lower the levels of IL-1β and block TNF-α, 2 cytokines essential to the immune dysfunction found in depression.23
Clinically, certain curcumin preparations have shown to be as effective in depression as fluoxetine, with no adverse effects.22 It's been associated with greater improved outcomes on the Inventory of Depressive Symptomatology when compared with placebo, with greater efficacy in atypical depression.22 Patients treated with curcumin also have shown improvement at About six weeks both in the Beck Depression Inventory and a healthcare facility Anxiety and Depression Scale scores.23 Bioavailability among preparations remains controversial in the literature. Dosage appears to range around 1000 mg daily during these trials, and seems to require about 1 month for sustained mood improvement.
Omega-3 Fatty Acids
The literature has presented some contradictory results regarding the efficacy of omega-3 polyunsaturated fatty acids and omega-3 fatty acids for depression.24 This is probably because of the wide variability of study populations, dosage, ratio of eicosapentaenoic acid to docosahexaenoic acid , and poor study-design . Despite this, a 2023 comprehensive meta-analysis of randomized numerous studies found significant clinical benefit of PUFA treatment compared to placebo for patients with proper diagnosis of MDD, as well as for depressive patients without this diagnosis.25 A 2011 meta-analysis of randomized controlled trials, involving as many as 916 participants with despression symptoms or bpd, discovered that omega-3 fatty acids supplements containing >60% EPA produced benefit versus supplements with <60% EPA, suggesting a greater role for EPA than DHA in depression and bipolar disorder.26
Elevated glucocorticoids happen to be associated with both increased glutamatergic neurotransmission and decreased gray matter in individuals with depression. PUFAs have been shown to protect glutamatergic neurotransmission from hyperactivation induced by stress and glucocorticoids.27 This presents a probable point to prevent depression onset when confronted with stressful stimuli. In addition, animal research has demonstrated increased serotonergic neurotransmission within the hippocampus when supplemented with omega-3 fatty acids.27
Mindfulness-Based Therapy
Mindfulness and meditation have been extensively studied across populations and types of conditions. In summary, mindfulness has been linked to outcome improvements, in terms of symptom reduction, biological markers, and neuroplasticity.28 The primary outcomes in which improvements are reported have been in decreasing depression, anxiety, pain, and illness-related distress.28 A particular meta-analytic review found effectiveness for mindfulness-based therapy across a number of conditions.29 Within this review, MBT significantly reduced anxiety symptoms among those by having an panic attacks, and seemed to be effective for reducing depressive symptoms in individuals with an analysis of depression.29 The result size was considered large for both of those instances. Two brain areas necessary to focus, self-awareness, and emotional regulation – the dorsal medial prefrontal cortex and anterior cingulate cortex – have shown to be activated during mindfulness meditation.30 In addition, structural MRI methods have produced a greater cortical thickness of the ACC in experienced meditators versus control subjects,30 the exact opposite pattern noticed in those with depression.
HPA Axis Support
Another large area of research for depression prevention and treatment is lifestyle, nutritional, and botanical medicine for sympathetic activation and HPA-axis feedback dysfunction. For a well-written and well-researched overview of neuroendocrine assessment and treatment of HPA disruption, see Dr Lise Alschuler's “Optimizing the HPA Axis” in the August 2023 issue of NDNR.31
Basic Action Steps
This research creates an important foundation that stem 3 important action steps: 1) comprehending the complexity of depression and mood disorders as structural and metabolic chronic diseases involving multiple systems and pathways; 2) offering evidence-based first-line therapies for long-term outcome improvement; and three) conceptualizing individualized treatment intends to turn back span of the psycho-neuro-endo-immunological cascades of depression and other mood disorders.

